Cumberland Pharmaceuticals Inc - ESG Rating & Company Profile powered by AI
Cumberland Pharmaceuticals Inc - ESG Rating & Company Profile powered by AI
This webpage is a free Sustainability assessment covering Cumberland Pharmaceuticals Inc. Comprehensive ESG assessment of Cumberland Pharmaceuticals Inc can be reached by registering for free. This assessment of Cumberland Pharmaceuticals Inc uses intelligence from across the internet and also from public documents by Cumberland Pharmaceuticals Inc.
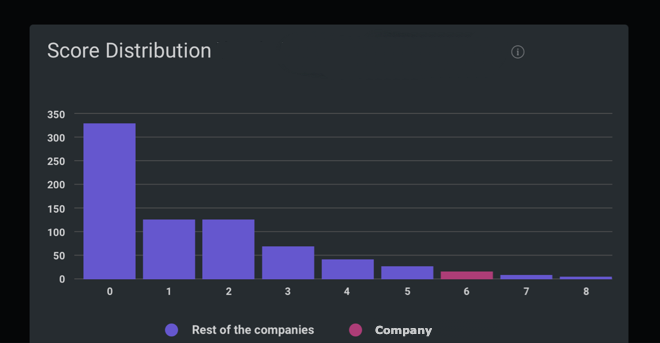
Cumberland Pharmaceuticals Inc in the Pharmaceuticals industry gained a UN SDG ESG Transparency Score of 4.0; made up of an environmental score of 4.0, social score of 2.7 and governance score of 5.3.
4.0
Medium ImpactEnvironmental
Social
Governance
Peer Group Comparison
| Rank | Company | SDG Transparency Score ⓘ | Performance |
|---|---|---|---|
| 1 | Caladrius Biosciences Inc | 8.0 | High |
| 1 | Can Fite Biopharma Ltd | 8.0 | High |
| 989 | Spectrum Pharmaceuticals Inc | 4.1 | High |
| 989 | Tricida Inc | 4.1 | High |
| 1004 | Cumberland Pharmaceuticals Inc | 4.0 | High |
| 1004 | Can B Corp | 4.0 | High |
| 1004 | CLS Holdings USA Inc | 4.0 | High |
| ... | ... | ... | |
| 1970 | Zyden Gentec Ltd | 0.0 | Low |
| 1970 | iNtRON Biotechnology Inc | 0.0 | Low |
| ... | ... | ... |
Frequently Asked Questions
Does Cumberland Pharmaceuticals Inc have an accelerator or VC vehicle to help deliver innovation?
Does Cumberland Pharmaceuticals Inc disclose current and historical energy intensity?
Does Cumberland Pharmaceuticals Inc report the average age of the workforce?
Does Cumberland Pharmaceuticals Inc reference operational or capital allocation in relation to climate change?
Does Cumberland Pharmaceuticals Inc disclose its ethnicity pay gap?
Does Cumberland Pharmaceuticals Inc disclose cybersecurity risks?
Does Cumberland Pharmaceuticals Inc use carbon offsets or credits exclusively for residual emissions (typically less than ~0.5–5% of total emissions)?
Does Cumberland Pharmaceuticals Inc offer flexible work?
Does Cumberland Pharmaceuticals Inc have a long term incentive (LTI) executive compensation plan based on a measure of return on capital?
Does Cumberland Pharmaceuticals Inc disclose the number of employees in R&D functions?
Does Cumberland Pharmaceuticals Inc plan to change its portfolio composition to lower the emissions intensity of its energy mix (e.g., by shifting from oil to gas, or by adding lower-carbon options like hydrogen, e-fuels, bioenergy, etc.)?
Does Cumberland Pharmaceuticals Inc conduct supply chain audits?
Does Cumberland Pharmaceuticals Inc disclose incidents of non-compliance in relation to the health and safety impacts of products and services?
Is there a statment that there is no plan to expand their cement production? (for example: 'We have no current plans to add additional cement making capacity')
Does Cumberland Pharmaceuticals Inc conduct 360 degree staff reviews?
Does Cumberland Pharmaceuticals Inc disclose the individual responsible for D&I?
Does Cumberland Pharmaceuticals Inc disclose current and historical air emissions?
Is there a statment that there is no plan to expand their coal usage? (for example: 'We have no current plans to add additional coal powered electricity generation')
Is executive remuneration linked to climate performance?
Does the Board describe its role in the oversight of climate-related risks and opportunities?
Does Cumberland Pharmaceuticals Inc disclose current and / or historical scope 2 emissions?
Does Cumberland Pharmaceuticals Inc disclose water use targets?
Does Cumberland Pharmaceuticals Inc have careers partnerships with academic institutions?
Did Cumberland Pharmaceuticals Inc have a product recall in the last two years?
Does Cumberland Pharmaceuticals Inc disclose incidents of discrimination?
Does Cumberland Pharmaceuticals Inc allow for Work Councils/Collective Agreements to be formed?
Has Cumberland Pharmaceuticals Inc issued a profit warning in the past 24 months?
Does Cumberland Pharmaceuticals Inc disclose parental leave metrics?
Does Cumberland Pharmaceuticals Inc disclose climate scenario or pathway analysis?
Does Cumberland Pharmaceuticals Inc disclose current and / or historical scope 1 emissions?
Does Cumberland Pharmaceuticals Inc explicitly state that carbon offsets or credits are separate from its emissions-reduction progress or that they are not counted toward its emissions-reduction targets?
Are Operating Expesnses linked to emissions reduction?
Does Cumberland Pharmaceuticals Inc disclose the pay ratio of women to men?
Does Cumberland Pharmaceuticals Inc support suppliers with sustainability related research and development?
Does Cumberland Pharmaceuticals Inc disclose the number of operations that have been subject to human rights reviews or human rights impact assessments?
Does Cumberland Pharmaceuticals Inc reflect climate-related risks in its financial statements?
Is there a statment that there is no plan to expand their carbon intensite energy assets? (for example: 'We have no current plans to carry out further drilling for oil,')
Is Cumberland Pharmaceuticals Inc involved in embryonic stem cell research?
Does Cumberland Pharmaceuticals Inc disclose GHG and Air Emissions intensity?
Does Cumberland Pharmaceuticals Inc disclose its waste policy?
Does Cumberland Pharmaceuticals Inc report according to TCFD requirements?
Does Cumberland Pharmaceuticals Inc plan to mitigate emissions from future new production assets through measures such as electrifying equipment, carbon capture and storage, repurposing waste gas, methane leak detection and repair, eliminating flaring, etc.?
Does Cumberland Pharmaceuticals Inc disclose its policies for bribery, corruption, whistle-blower, conflict of interest?
Does Cumberland Pharmaceuticals Inc disclose energy use targets?
Does Cumberland Pharmaceuticals Inc disclose its Renewable Energy targets?
 Subscription required
Subscription requiredAre emissions metrics verified by STBi?
 Subscription required
Subscription requiredDoes Cumberland Pharmaceuticals Inc have a policy relating to cyber security?
Have a different question?
Potential Risks for Cumberland Pharmaceuticals Inc
These potential risks are based on the size, segment and geographies of the company.
Cumberland Pharmaceuticals Inc., a specialty pharmaceutical company, focuses on the acquisition, development, and commercialization of prescription products for hospital acute care, gastroenterology, rheumatology, and oncology in the United States and internationally. The company offers Acetadote, an injection for the treatment of acetaminophen poisoning; Caldolor, an injection for the treatment of pain and fever; Kristalose, a prescription laxative oral solution for the treatment of chronic and acute constipation; Omeclamox-Pak for the treatment of Helicobacter pylori infection and duodenal ulcer disease; Vaprisol, an injection for treating euvolemic and hypervolemic hyponatremia; and Vibativ, an injection for the treatment of certain serious bacterial infections, including hospital-acquired and ventilator-associated bacterial pneumonia, as well as complicated skin and skin structure infections. It also develops RediTrex injection for the treatment of active rheumatoid, juvenile idiopathic, and severe psoriatic arthritis, as well as disabling psoriasis. In addition, the company is developing ifetroban, a product candidate that is in phase II clinical trial for the treatment of aspirin-exacerbated respiratory disease, systemic sclerosis, and duchenne muscular dystrophy; and has completed phase II clinical trial for the treatment of hepatorenal syndrome and portal hypertension. Further, it develops a clinical program for the use of ifetroban to treat progressive fibrosing interstitial lung diseases; and a product candidate that is in Phase II clinical trial for cholesterol reducing agent to use in the hospital setting. The company was incorporated in 1999 and is headquartered in Nashville, Tennessee.